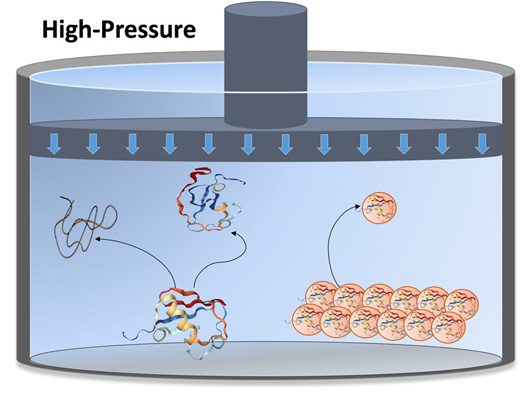
High-pressure NMR
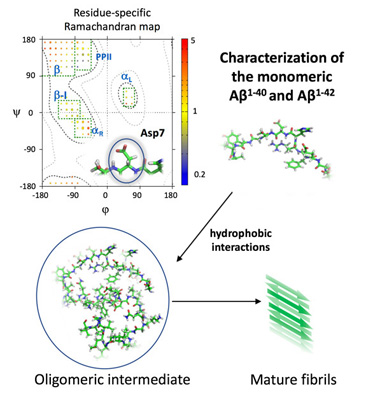
Scaffold proteins
and disordered peptides
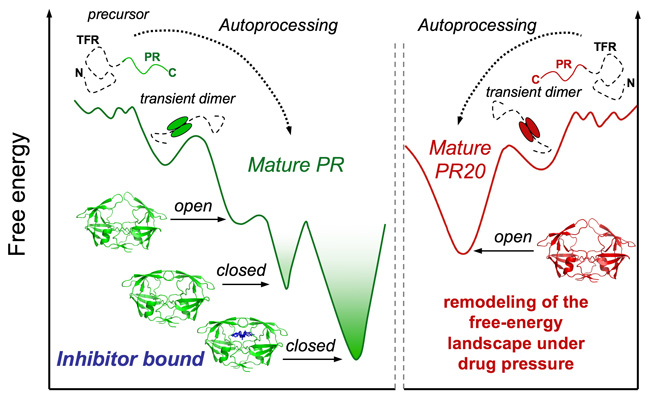
Evolution of
viral proteins
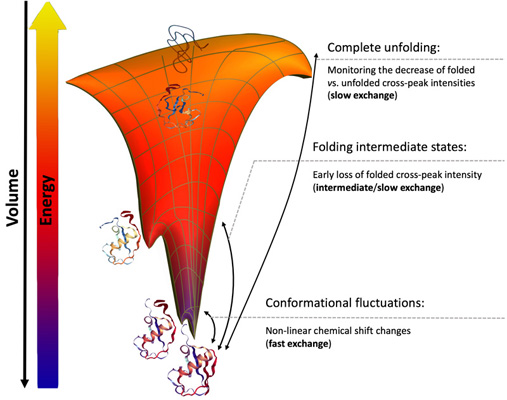
We study the mechanisms of folding, activation, fibrillation and evolution of proteins involved in neurodegenerative and infectious diseases.
We use a combination of Nuclear Magnetic Resonance (NMR) spectroscopy, X-ray crystallography, molecular dynamics simulations and bioinformatics techniques to characterize the structural and functional properties of these proteins at an atomistic level.
The detailed characterization of these complex mechanisms provides a molecular basis for the development of new therapeutic approaches.
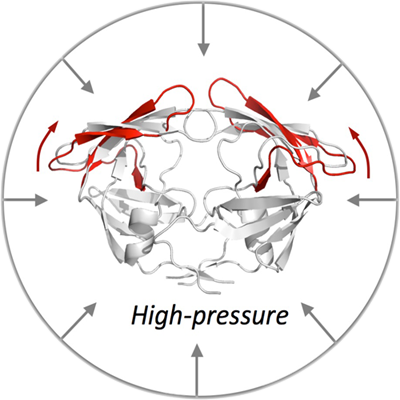

New website: The Structural Biology portal is now live!
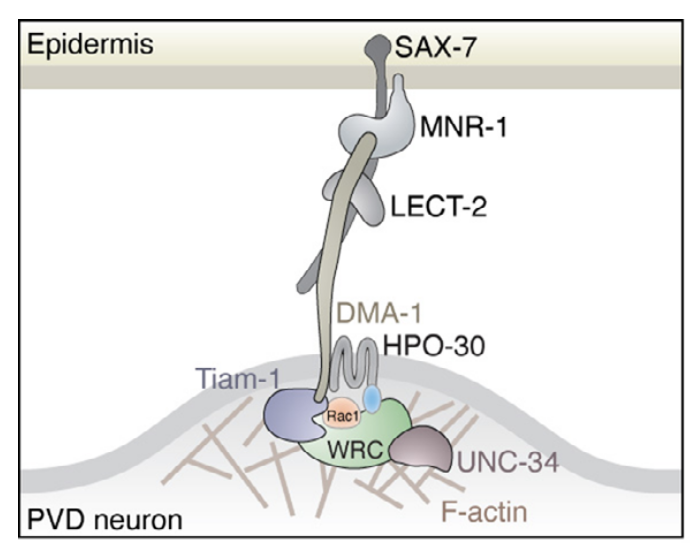
Our work in collaboration with Dr. Stone Chen at ISU has been published in eLife!
Our new publication is available online: "Intricate coupling between the transactivation and basic-leucine zipper domains governs phosphorylation of transcription factor ATF4 by casein kinase 2"